UNLOCK YOUR POTENTIAL WITH VIRAL SENSITIZERS VSEs™
Vaccines and advanced therapies hold great promise to protect and save human life, yet their full potential is often limited by the cost of manufacturing.
These therapies face fundamental manufacturing and therapeutic challenges due to cellular antiviral defenses.
Our proprietary and pioneering Viral Sensitizers (VSE™) unlock your potential to increase yield and effectiveness while reducing cost.
Virica is the first company to develop and commercialize VSEs to solve the real-world challenges facing the industry.
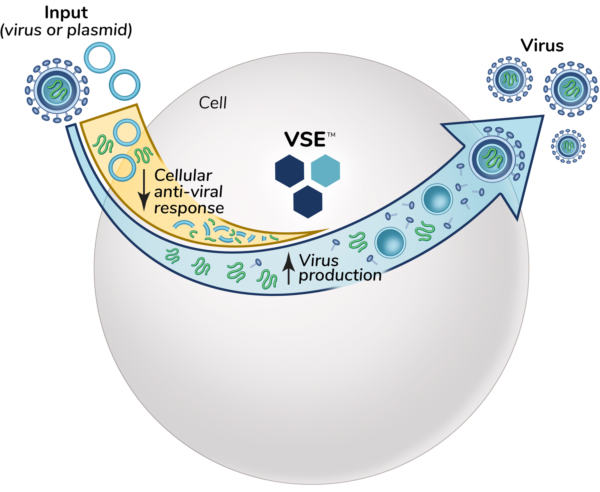
HOW DO VSEs WORK?
VSEs are proprietary small molecules that that improve viral yields and effectiveness by reducing antiviral defenses on a cellular level. VSE work by suppressing anti-viral defenses found in manufacturing cells. VSEs do this by transiently altering cell signaling, which allows the virus to infect, transduce, and replicate better.
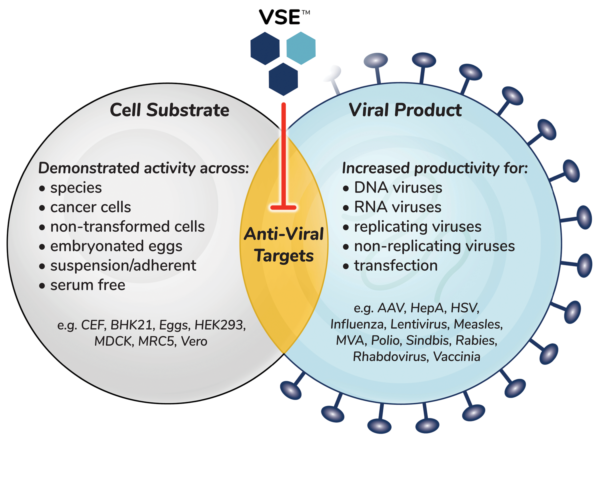
We have a library of over 100 VSEs. Each are different from one another, so each attenuate the antiviral defense in different ways. For this reason, VSEs are sometimes more effective in combination. VSEs work in a broad variety of viruses and commonly used cell substrates and can be optimized for each specific cell/virus combination.
APPLICATIONS
VSEs have been evaluated in a broad range of gene therapy and vaccine applications. To achieve meaningful virus titers in vaccine applications or higher yields of viral vectors in gene therapies, you rely on living cells, grown at scale. Our VSE technology allows you to achieve the highest viral vector performance. We help you achieve your goals whether they be higher yield, effectiveness or scale.

Our proprietary combination of VSE compounds robustly enhances the transfection-based production (3rdgeneration) of lentivirus in adherent HEK293T cells by 5-fold


Addition of a VSE formulation to a small-scale culture improved MVA yield by up to 10X across multiple strains. In a scale up model using an iCells Nano (0.53 – 4.0m2), addition of the VSE formulation improved yield by 4X without any optimization, suggesting that scaling up of the technology is feasible.

VSE formulation originally validated in Ambr® 15 scale was successfully scaled to a 5L suspension bioreactor (3.75L working volume).
VSE addition resulted in multi-fold enhancement in total capsids (ELISA), genomic titer (ddPCR) and % full AAV particles.
INCORPORATING VSEs IN YOUR PROCESS
VSEs can be administered at or around the time of infection or transfection to attenuate the antiviral defenses. VSEs are metabolized rapidly by producer cells (≤24hrs) and are cleared prior to downstream processing of the final product by standard purification methods.

ANALYTICS AND REGULATORY SUPPORT
Virica provides all the support needed to help meet the necessary regulatory requirements for the incorporation of our VSEs into your processes.
Virica has toxicological information on key VSEs to support regulatory filings, if required
GMP-compliant VSE compounds will be made available to customers, if required, with a 3-month lead time
GMP Enabled

Analytical assays for tracking VSE molecules during manufacturing. Top left panel: Schematic of multiple reaction monitoring approach (MRM). Top right panel: Data showing limit of detection for our VSE is in the femtomole range using MRM. Bottom panel: VSE Molecules are easily purified away using standard methods. Purified MVA preparations that are grown in the presence of VSEs (B) show no trace of VSEs compared to control purified MVA preparations that are not grown in the presence of VSEs but are spiked with VSEs prior to MRM-Mass Spectrometry (A).
